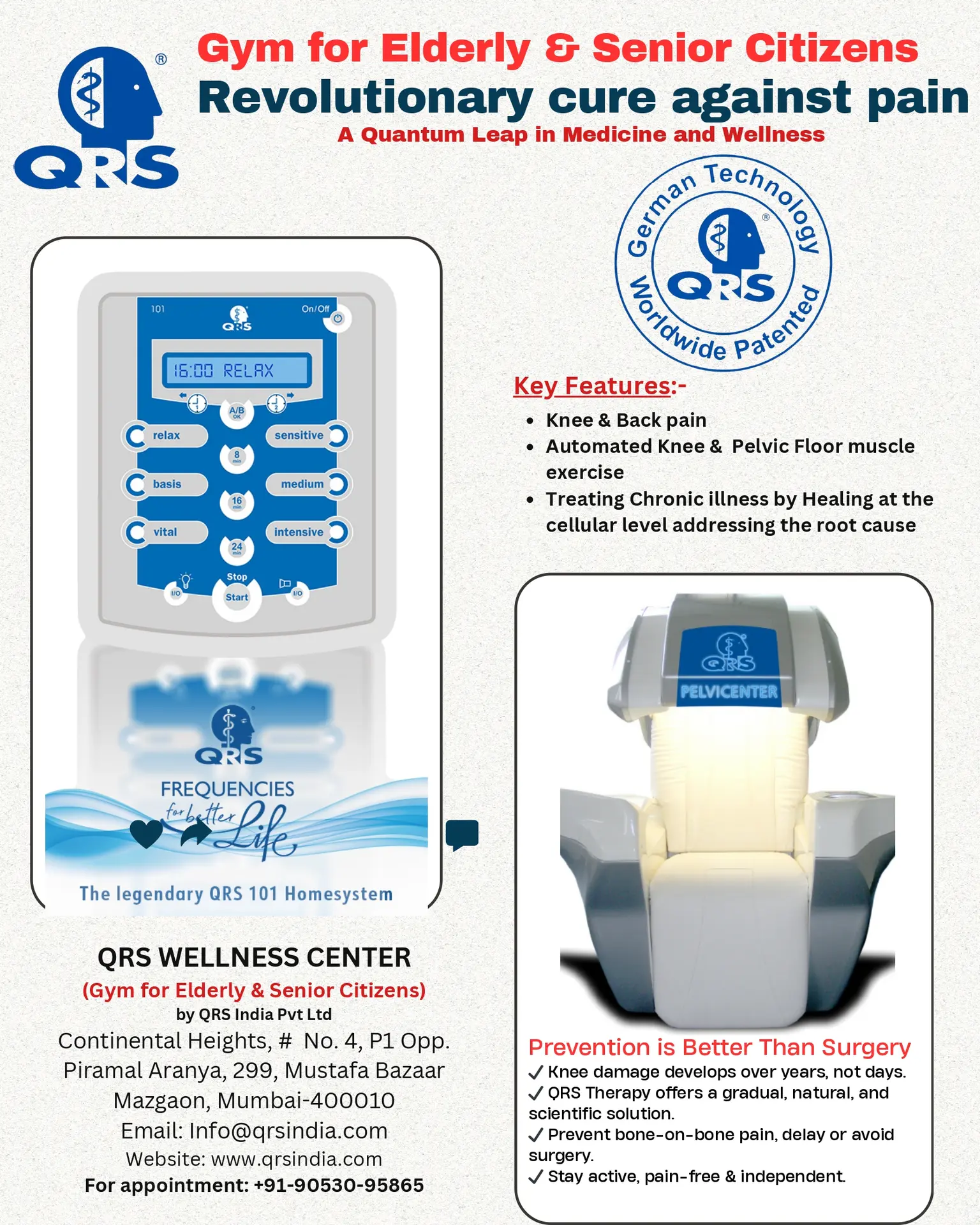
Certifications & Quality
Uncompromising quality standards and international certifications that guarantee your safety and therapeutic effectiveness
German Engineering Excellence
QRS systems are manufactured in Germany under the most stringent quality control standards in the medical device industry. Every component undergoes rigorous testing to ensure consistent performance and patient safety.
Our commitment to quality is reflected in our comprehensive certifications and compliance with international medical device regulations, making QRS the trusted choice for healthcare professionals worldwide.
Medical Device Directive Compliance
Full compliance with European MDD 93/42/EEC
ISO Quality Management
ISO 13485:2016 certified manufacturing
International Recognition
Approved in 100+ countries worldwide
International Certifications
Comprehensive regulatory approvals ensuring global safety and efficacy standards
CE Marking
European Conformity marking certifies that QRS meets EU health, safety, and environmental protection standards for medical devices.
Class: IIa Medical Device
Directive: MDD 93/42/EEC
ISO 13485:2016
International standard for quality management systems in medical device design, development, production, and distribution.
Scope: Full Manufacturing Process
Audit: Annual Third-Party Verification
TÜV Certification
German Technical Inspection Association certification for product safety, quality, and electromagnetic compatibility.
Testing: EMC & Safety Standards
Standard: IEC 60601-1-2
FCC Approval
US Federal Communications Commission approval for electromagnetic emission compliance and interference prevention.
Class: Class B Device
Part: CFR 47 Part 15
Health Canada
Canadian health authority approval for medical device safety and efficacy in the Canadian market.
License: Medical Device License
Class: Class II Device
UKCA Certification
UK Conformity Assessed marking demonstrating compliance with United Kingdom medical device regulations.
Class: Medical Device
Regulation: UK MDR 2002 (as amended)
Quality Control Process
Every QRS system undergoes rigorous testing at multiple stages of production
Design Validation
Engineering design validation against technical specifications and safety requirements
- Circuit design verification
- Safety component testing
- Performance validation
Component Testing
Individual component testing for quality, durability, and performance specifications
- Incoming material inspection
- Electronic component testing
- Applicator quality check
Assembly Quality
Precision assembly process with continuous quality monitoring and testing
- Assembly line inspection
- Functional testing
- Calibration verification
Final Inspection
Comprehensive final inspection and testing before packaging and shipment
- Complete system testing
- Safety compliance check
- Quality certification
Safety Standards Compliance
QRS systems exceed the most stringent international safety standards for medical devices, ensuring complete protection for users while delivering optimal therapeutic benefits.
IEC 60601-1
Medical electrical equipment safety standards
IEC 60601-1-2
Electromagnetic compatibility requirements
IEC 62304
Medical device software lifecycle processes
ISO 14971
Medical device risk management
Temperature Protection
Automatic thermal shutdown prevents overheating
Electrical Safety
Double insulation and ground fault protection
Session Control
Automatic timer prevents excessive exposure
EMF Protection
Contained field design prevents interference
Research Documentation
Access our comprehensive research documentation and clinical studies
Experience Certified Quality
When quality and safety matter most, trust the only PEMF system with comprehensive international certifications
📞 Call: +91-740-423-4242 | 📧 Email: info@qrsindia.com